Biosimilar Market - Global Market Share, Trends, Analysis and Forecast, 2023-2032
The global biosimilar market size was estimated to be US$ 29.5 billion in 2021 and is expected grow at a CAGR of 11.5% between 2023 to 2032.
A biosimilar is defined as “a biological product that is highly similar to and has no clinically meaningful differences from an existing FDA-approved reference product.” To manufacture a biosimilar, the structure and function of both the reference product and biosimilar are extensively analyzed and demonstrated by the manufacturer. For characterization of biosimilars, properties such as purity, chemical identity and bioactivity are compared. These comparative tests along with other information are used by the manufacturer to prove that the biosimilar is highly similar to the reference product.
Biosimilar market growth is mainly driven by the rising incidences of chronic diseases and increasing demand for their cost-effectiveness. Regulations favoring biosimilar adoption in different countries and easier regulatory approval processes are major factors driving market growth.
Biosimilars are prescribed for treating a wide range of conditions such as type 1 and type 2 diabetes and growth hormone disorders, post-menopausal osteoporosis, anemia, kidney failure, cancer, rheumatoid arthritis, infectious disorders and psoriasis.
The key target therapeutic areas for which various biosimilars are in the pipeline include oncology, autoimmune disorders, diabetes, and hepatitis. Besides these, biosimilars can be developed for other chronic disorders, such as meningitis, breast cancer, adult T-cell leukemia, obesity, hypertension, and hepatitis E.
Increasing demand for biosimilar drugs due to their cost-effectiveness
Since biosimilars have lower R&D costs as compare to novel biologics, this results in lower cost of biosimilars which ultimately results in cost-savings and access to highly effective treatment for patients. Due to price competition among manufacturers, lower-priced biosimilars tend to have a downward effect on the prices of reference biologics. This substantial cost-to-benefit ratio offered by biosimilar drugs is expected to increase their demand in the coming years.
Expiring patents for blockbuster biologic
Most of the current best-selling biologics are set to lose their patent protection in the coming years which will create new opportunities for biosimilar drugs. By year 2023, patents of nearly 20 oncology biologics will expire, which could possibly lead to more biosimilars in oncology.
Challenges in the global biosimilar market:
Complexities in manufacturing
Developing biosimilars is a highly complex and costly process that requires significant investments, clinical trial expertise, quality systems, scientific standards and technical capabilities. Biosimilar manufacturers are required to invest in clinical trials and post-approval safety monitoring programs that are similar to that of original innovator companies, unlike in the development of generic medicines.
The ability to control variability during the manufacturing process is another key challenge in manufacturing biosimilars, wherein the end products are similar to their biological products. Regulatory authorities may require additional preclinical and clinical data to prove that the manufacturing process has not impacted the safety or the efficacy of the product and there is zero variability between the biosimilar and the novel biologic.
Excess Competition
Biosimilar manufacturers not only have to compete with other biosimilar manufacturers but also with the original biologic manufacturer. As new biosimilar products enter the market, the original biologic manufacturer can defend from the competition using means like launch of reformulated drugs, second-generation products, supporting devices, dosing improvements and competing on prices.
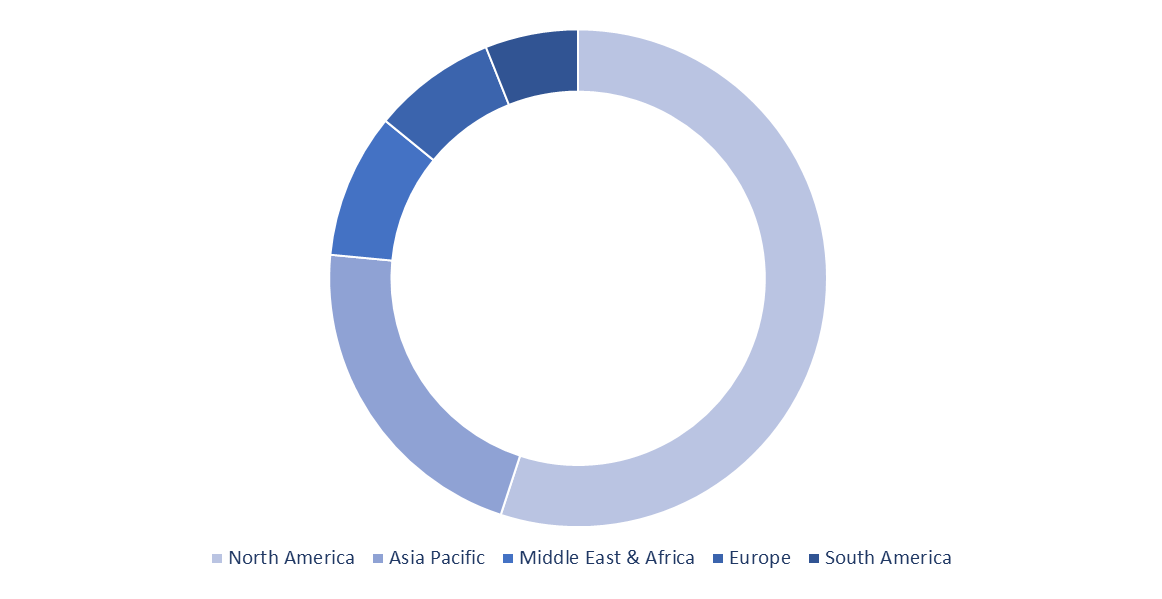
Biosimilar Market Share Analysis, by Geography (2021)
The major players covered in the biosimilar market report are Novartis AG, Pfizer Inc., Dr. Reddy’s Laboratories Ltd., Amgen Inc., Eli Lilly and Company, Teva Pharmaceutical Industries Ltd., Fresenius SE & Co., STADA Arzneimittel AG, Boehringer Ingelheim, Gedeon Richter PLC, Celltrion, Samsung Biologics, Coherus BioSciences, Biocon Limited, Viatris Inc., Amega Biotech, Apotex Inc., Biocad, mAbxience, Probiomed S.A. De C.V., Fujifilm Kyowa Kirin Biologics Co Ltd., Intas Pharmaceuticals Ltd., Thermex, Reliance Life Sciences, Kashiv Biosciences and others.
Global Biosimilar Market Segmentation:
By Product
- Monoclonal Antibodies
- Infliximab
- Trastuzumab
- Rituximab
- Adalimumab
- Other monoclonal antibodies (bevacizumab, cetuximab, ranibizumab, denosumab, and eculizumab)
- Insulin
- Granulocyte Colony-Stimulating Factor
- Erythropoietin
- Recombinant Human Growth Hormone
- Etanercept
- Follitropin
- Teriparatide
- Interferons
- Enoxaparin Sodium
- Glucagon
- Calcitonin
By Indication
- Oncology
- Inflammatory & Autoimmune Disorders
- Chronic Diseases
- Blood Disorders
- Growth Hormone Deficiency
- Infectious Diseases
- Other Indications (infertility, hypoglycemia, postmenopausal osteoporosis, chronic kidney failure, and ophthalmic diseases)
By Geography
- North America
- United States
- Canada
- Rest of North America
- Europe
- Germany
- United Kingdom
- Italy
- France
- Spain
- Rest of Europe
- Asia Pacific
- Japan
- India
- China
- Australia
- Rest of Asia Pacific
- Middle East & Africa
- UAE
- Saudi Arabia
- South Africa
- Rest of the Middle East & Africa
- South America
- Brazil
- Rest of South America