The global in vitro diagnostics (IVD) quality control market size was estimated to be US$ 1.2 billion in 2022 and is expected to reach US$ 1.79 billion by 2032 at a CAGR of 4.1%.
In-vitro diagnostics are assessments that can discover illnesses, disorders, or contagions. Some of the tests are used in the laboratory or other health professional locations and other tests are for patients to conduct at home. They comprise medical devices and equipment used to execute assessments on specimens so as to assist to discover the contagion, to detect a medical disorder, to avert illness, and to monitor medication treatments.
The global IVD quality control market is mainly propelled by the increased number of certified clinical labs, growing demand for third-party quality controls, and escalating commonness of chronic and contagious illnesses. An increasing number of certified clinical labs worldwide and the presence of promising governing organizations are anticipated to be the main factors expanding the market growth. Diagnostic labs are observing fast advancement due to the huge predominance of disorders, such as diabetes, Cardiovascular Diseases (CVDs), and contagious illnesses. Many private, as well as public labs, are undergoing laboratory accreditation procedures to satisfy industry criteria, enhance their procedural volume, and lure more patients.
The North America led the global IVD quality controls market in 2019, and is projected to remain in leading position throughout the evaluation period, as a result of surge in demand for technically superior multi-analytical controls and strict directives for using controls. The APAC region is estimated to be the rapidly developing region over the assessment timeline as the region has a huge potential for this market because of the growing number of product manufacturing corporations.
Besides, the Asia Pacific Federation of Clinical Biochemistry and Laboratory Medicine has been boosting the application of IVDs. Therefore, an increase in consciousness for initial and accurate diagnosis promotes the product demand and ultimately the IVD quality control market in the region. Also, the presence of key regulatory authorities in the region, such as the Ministry of Health, Labor and Welfare (MHLW), Pharmaceuticals and Medical Devices Evaluation Agency (PMDA), and Ministry of Agriculture and Fisheries and Food, will support the market growth.
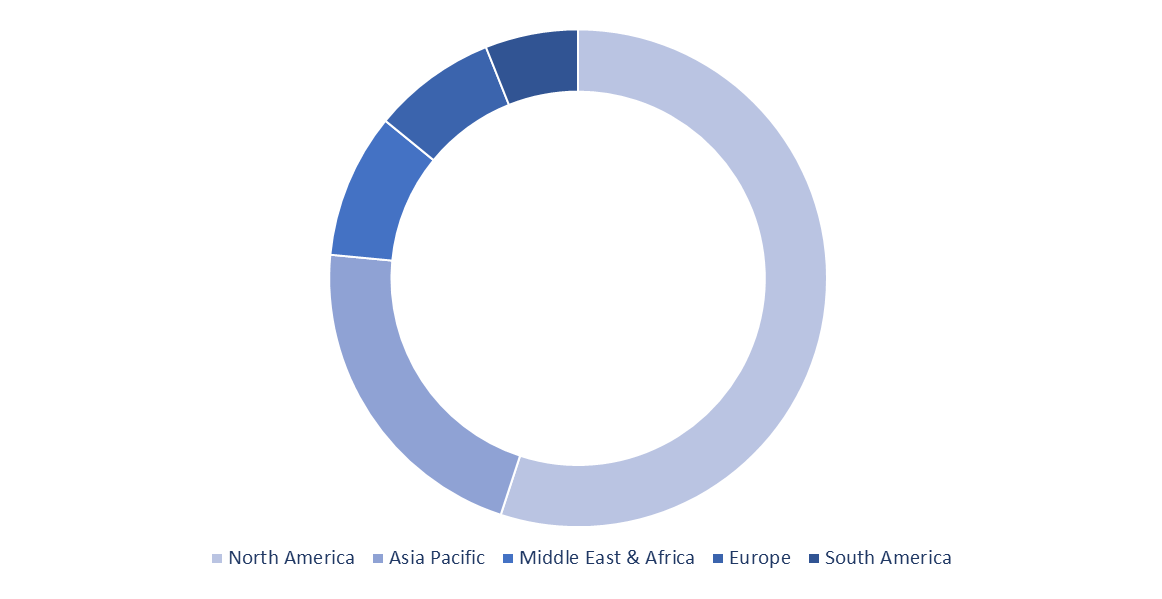
In Vitro Diagnostics (IVD) Quality Control Market Share Analysis, by Geography (2022)
The report titled “In Vitro Diagnostics (IVD) Quality Control Market - Global Market Share, Trends, Analysis and Forecasts, 2023-2032” wherein 2021 is the historic period, 2022 is the base year, and 2023 to 2032 is forecast period. Additionally, the study takes into consideration the competitive landscape, wherein the report would provide company overview and market outlook for leading players in the global In Vitro Diagnostics (IVD) Quality Control Market.
Furthermore, the report would reflect the key developments, global & regional sales network, business strategies, research & development activities, employee strength, and key executive, for all the major players operating in the market.
The global In Vitro Diagnostics (IVD) Quality Control market is segmented depending on application, type, end-use, and geography. By application, the IVD quality control market is divided into Immunochemistry, Hematology, Clinical Chemistry, Molecular Diagnostics, Coagulation, Microbiology, and Others. The type segment is further divided into quality control, quality assurance services, data management. Based on end-use the market is segmented into hospitals, labs, home-care, and others. Based on geography, the global In Vitro Diagnostics (IVD) Quality Control market is segmented into North America, Europe, Asia Pacific, Middle East & Africa, and South America. North America is sub-segmented into the United States, Canada and Rest of North America. Europe is sub-segmented into Germany, the United Kingdom, Belgium, Spain, and Rest of Europe. Asia Pacific is sub-segmented into China, Japan, India, Indonesia, Australia, South Korea, Taiwan, and Rest of Asia Pacific. Middle East & Africa is sub-segmented into Saudi Arabia, the UAE, and Rest of Middle East & Africa. South America is sub-segmented into Brazil and Rest of South America.
The research provides in-depth analysis of prominent players holding majority share of the global market with a focus on all operating business segment and would identify the segment of the company focusing on In Vitro Diagnostics quality control. Further, market share of prominent companies in the global In Vitro Diagnostics (IVD) Quality Control Market would also be estimated.
The study takes into consideration the key competitive information such as business strategy, product portfolio, key development, swot analysis, and research and development focus of all the In-vitro diagnostics companies. The global In Vitro Diagnostics (IVD) Quality Control Market study would take into consideration the participants engaged throughout the supply chain and value chain of the market, along with their contribution.
Product portfolio would focus on all the products under the In-vitro diagnostics business segment of the company. Similarly, the recent development section would focus on the latest developments of company such as strategic alliances and partnerships, merger and acquisition, new product launched and geographic expansion in the global In Vitro Diagnostics (IVD) Quality Control Market.
Major players active in the global In Vitro Diagnostics (IVD) Quality Control Market Thermo Fisher Scientific, Inc., Bio-Rad Laboratories, Inc., Sero AS, Siemens Healthcare GmbH, Bio-Techne, Qiagen N.V., Sysmex Corp., Abbott Laboratories, Inc., Becton, Quidel Corp., Dickinson, and Company (BD), bioMerieux, Inc., Alere, Inc., Hologic, Inc. (Gen-Probe), and Roche Diagnostics.
By Type
By Application
By End Use
By Geography :
The in vitro diagnostics quality control market size is expected to reach US$ 1.79 billion by 2032.
The in vitro diagnostics quality control market is expected grow at a CAGR of 4.1% from 2023 to 2032.
The in vitro diagnostics quality control market size was estimated to be US$ 1.2 billion in 2022.
Major participants in the In Vitro Diagnostics (IVD) Quality Control industry comprise Thermo Fisher Scientific, Inc., Bio-Rad Laboratories, Inc., Sero AS, Siemens Healthcare GmbH, Bio-Techne, Qiagen N.V., Sysmex Corp., Abbott Laboratories, Inc., Becton, Quidel Corp., Dickinson, and Company (BD), bioMerieux, Inc., Alere, Inc., Hologic, Inc. (Gen-Probe), and Roche Diagnostics..
The base year considered in In Vitro Diagnostics (IVD) Quality Control market report is 2022.
Copyright © 2025 Same Page Management Consulting Pvt. Ltd. (insightSLICE) | All Rights Reserved
